One of the easiest and most cost-effective ways to reduce NOx and improve fuel efficiency is to measure oxygen (O₂) and combustibles (CO+H2 or often abbreviated as COe for carbon monoxide equivalent). NOx emissions from combustion sources react to form smog and acid rain and contribute to the formation of ground-level ozone and fine particles. For this reason, most countries have regulations limiting the amount of NOx that can be emitted from combustion plants. In the USA, the Transport Rule establishes a strict program for NOx reductions in many areas. This makes NOx control an important consideration for plant operators.
NOx emissions can be controlled by limiting the amount of available O₂, which can combine with nitrogen (N₂) to form nitric oxide (NO). Because the mixing of air and fuel can never be perfect, some excess air is always required to ensure complete combustion. By limiting this excess amount of air, less NOx is likely to form. However, if too little excess air is available, the combustibles in the flue gas rise dramatically. Knowing the O₂ and combustibles concentrations in the flue gas allows the amount of excess air to be controlled, while maintaining good combustion efficiency. The optimum control point to minimize NOx emissions and efficiency losses is shown in Figure 1.
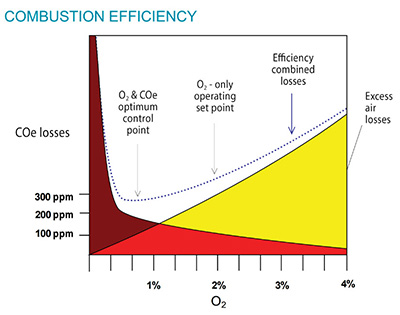
Figure 1. COe vs. excess O₂ showing efficiency losses (this figure for illustration purposes only)
The optimum operating point can vary by process as many factors come into play, including load conditions, age of equipment, fuel type and process conditions. By continuously monitoring the amount of O2 and combustibles in the flue gas, prompt adjustments can be made to maintain optimum burner conditions.
For more information on this application click here to view the related Application Note.